A) tertiary carbocation is more stable than primary carbocation
B) primary carbocation is more stable than tertiary carbocation
C) t-butanol has a higher boiling point
D) rearrangement takes place during dehydration of t-butanol.
Correct Answer: A
Solution :
During the reaction carbocations are formed. \[3{}^\circ \]carbocation is more stable than \[1{}^\circ \]carbocation, hence the dehydration in \[3{}^\circ \] alcohol proceeds faster than \[1{}^\circ \]alcohol.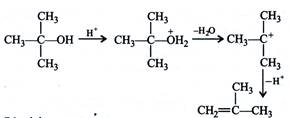
You need to login to perform this action.
You will be redirected in
3 sec
