A) \[I{{F}_{3}}\]
B) \[PC{{l}_{3}}\]
C) \[N{{H}_{3}}\]
D) \[B{{F}_{3}}\]
Correct Answer: D
Solution :
No. of e? pair\[=3+\frac{1}{2}[3-3]\]=0 No. of e? pair\[=3\]+0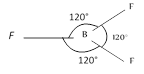 No. of atom bonded to the central atom \[=3\] In case of 3, 3 geometry is Trigonal planar.
No. of atom bonded to the central atom \[=3\] In case of 3, 3 geometry is Trigonal planar.
You need to login to perform this action.
You will be redirected in
3 sec
