A)
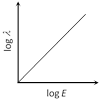
B)
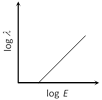
C)
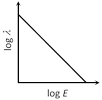
D)
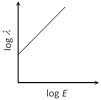
Correct Answer: C
Solution :
\[\lambda =\frac{h}{\sqrt{2mE}}=\frac{h}{\sqrt{2m}}\cdot \frac{1}{\sqrt{E}}\]. Taking log of both sides \[\log \lambda =\log \frac{h}{\sqrt{2}m}+\log \frac{1}{\sqrt{E}}\]Þ\[\log \lambda =\log \frac{h}{\sqrt{2}m}-\frac{1}{2}\log E\] \[\Rightarrow \log \lambda =-\frac{1}{2}\log E+\log \frac{h}{\sqrt{2m}}\] This is the equation of straight line having slope (?1/2) and positive intercept on log l axis.You need to login to perform this action.
You will be redirected in
3 sec
