A) \[{{P}_{3}}>{{P}_{1}},\,\,W>0\]
B) \[{{P}_{3}}<{{P}_{1}},\,\,W<0\]
C) \[{{P}_{3}}>{{P}_{1}},\,\,W<0\]
D) \[{{P}_{3}}={{P}_{1}},\,\,W=0\]
Correct Answer: C
Solution :
From graph it is clear that \[{{P}_{3}}>{{P}_{1}}\]. Since area under adiabatic process (BCED) is greater than that of isothermal process (ABDE). Therefore net work done \[W={{W}_{i}}+(-{{W}_{A}})\] \[\because \ {{W}_{A}}>{{W}_{i}}\] Þ \[W<0\]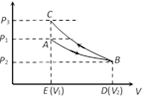
You need to login to perform this action.
You will be redirected in
3 sec
