A) \[2{{P}_{0}}A\]
B) \[{{P}_{0}}A\]
C) \[\frac{{{P}_{0}}A}{2}\]
D) \[4{{P}_{0}}A\]
Correct Answer: B
Solution :
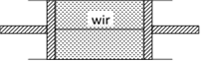
Volume of the gas is constant V = constant \ \[P\propto T\] i.e., pressure will be doubled if temperature is doubled \ \[P=2{{P}_{0}}\] Now let F be the tension in the wire. Then equilibrium of any one piston gives \[F=(P-{{P}_{0}})A=(2{{P}_{0}}-{{P}_{0}})A={{P}_{0}}A\]
Now let F be the tension in the wire. Then equilibrium of any one piston gives \[F=(P-{{P}_{0}})A=(2{{P}_{0}}-{{P}_{0}})A={{P}_{0}}A\]
You need to login to perform this action.
You will be redirected in
3 sec
