A)
X
Y
Z
\[PbS{{O}_{4}}\]
\[P{{b}_{2}}{{O}_{3}}\]
\[S{{O}_{3}}\]
B)
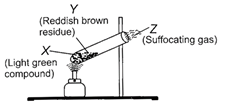
X
Y
Z
\[FeS{{O}_{4}}.\,7{{H}_{2}}O\]
\[F{{e}_{2}}{{O}_{3}}\]
\[S{{O}_{2}},\,S{{O}_{3}}\]
C)
X
Y
Z
\[N{{a}_{2}}S{{O}_{4}}.\,10{{H}_{2}}O\]
\[N{{a}_{2}}S{{O}_{4}}\]
\[S{{O}_{2}}\]
D)
X
Y
Z
\[Pb{{(N{{O}_{3}})}_{2}}\]
\[Pb{{O}_{2}}\]
\[N{{O}_{2}},\,{{N}_{2}}{{O}_{4}}\]
Correct Answer: B
Solution :
\[FeS{{O}_{4}}.7{{H}_{2}}O\xrightarrow{heat}\underset{\begin{smallmatrix} Ferrous \\ sulphate \end{smallmatrix}}{\mathop{FeS{{O}_{4}}}}\,+7{{H}_{2}}O\] \[\underset{\begin{smallmatrix} Ferrous \\ sulphate \end{smallmatrix}}{\mathop{2FeS{{O}_{4}}}}\,\xrightarrow{heat}\underset{\begin{align} & \operatorname{Re}ddish \\ & brown \\ & residue \\ & \,\,\,\,\,(Y) \\ \end{align}}{\mathop{F{{e}_{2}}{{O}_{3}}}}\,+\underbrace{\underset{\begin{smallmatrix} Sulphur \\ dioxide \end{smallmatrix}}{\mathop{S{{O}_{2}}}}\,+\underset{\begin{smallmatrix} Sulphur \\ trioxide \end{smallmatrix}}{\mathop{S{{O}_{3}}}}\,}_{(Z)}\] \[S{{O}_{2}}\]and \[S{{O}_{3}}\] are acidic in nature, turn blue litmus red as they react with water to form acids as follows : \[S{{O}_{2}}+{{H}_{2}}O\xrightarrow{{}}\underset{(Sulphurous\,acid)}{\mathop{{{H}_{2}}S{{O}_{3}}}}\,\] \[S{{O}_{3}}+{{H}_{2}}O\xrightarrow{{}}\underset{(Sulphurous\,acid)}{\mathop{{{H}_{2}}S{{O}_{4}}}}\,\]You need to login to perform this action.
You will be redirected in
3 sec
