| Directions : (Q. 16 to 20) | |||||||||||||||||||||||||||||||||||
| Case IV: Read the passage given below and answer the following questions. | |||||||||||||||||||||||||||||||||||
| In haloalkanes, when a nucleophile stronger than the halide ion approaches the positively charged carbon atom of an alkyl halide, the halogen atom along with its bonding electron pair gets displaced and a new bond with the carbon and the nucleophile is formed. These reactions are called nucleophilic substitution reactions. | |||||||||||||||||||||||||||||||||||
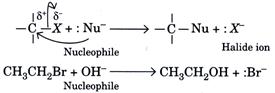 | |||||||||||||||||||||||||||||||||||
| In these reactions the atom or group of atoms which loses its bond from carbon and takes on an additional pair of electrons is called leaving group. Halide ions are good leaving groups. Some important nucleophilic substitution reactions of haloalkanes with common nucleophiles are given in the table below. | |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
| In the following questions (Q. No. 16-20), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage. |
| Assertion: Alkyl halides are hydrolysed to alcohols by moist silver oxide. |
| Reason: RCl is hydrolysed to ROH easily but reactions slow down on addition of KI. |
A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
C) Assertion is correct statement but reason is wrong statement.
D) Assertion is wrong statement but reason is correct statement.
Correct Answer: C
Solution :
Kl reacts with RCl to form RI. This RI molecule now hydrolysed easily to give ROH because alkyl iodide are more reactive than alkyl chloride. Thus, reaction becomes faster on addition of Kl.You need to login to perform this action.
You will be redirected in
3 sec
