A) 100 calorie
B) 0.01 kilocalorie
C) 716 calorie
D) 1 kilocalorie
Correct Answer: C
Solution :
Conversion of ice (0°C) into steam (100°C) is as follows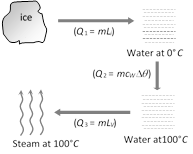 Heat required in the given process \[={{Q}_{1}}+{{Q}_{2}}+{{Q}_{3}}\] \[=1\times 80+1\times 1\times (100-0)+1\times 536=716\,cal\]
Heat required in the given process \[={{Q}_{1}}+{{Q}_{2}}+{{Q}_{3}}\] \[=1\times 80+1\times 1\times (100-0)+1\times 536=716\,cal\]
You need to login to perform this action.
You will be redirected in
3 sec
