A) n = 2 to n = 1
B) n = 4 to n = 3
C) n = 3 to n = 1
D) n = 4 to n = 2
Correct Answer: B
Solution :
From diagram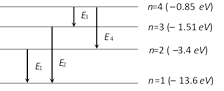 \[{{E}_{1}}=-13.6-(-3.4)=-10.2\,eV\] \[{{E}_{2}}=-13.6-(-1.51)=-12.09\,eV\] \[{{E}_{3}}=-1.51-(-0.85)=-0.66\,eV\] \[{{E}_{4}}=-3.4-(-1.51)=-1.89\,eV\] \[{{E}_{3}}\] is least i.e. frequency is lowest.
\[{{E}_{1}}=-13.6-(-3.4)=-10.2\,eV\] \[{{E}_{2}}=-13.6-(-1.51)=-12.09\,eV\] \[{{E}_{3}}=-1.51-(-0.85)=-0.66\,eV\] \[{{E}_{4}}=-3.4-(-1.51)=-1.89\,eV\] \[{{E}_{3}}\] is least i.e. frequency is lowest.
You need to login to perform this action.
You will be redirected in
3 sec
