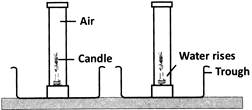
 What can be concluded from the above activity?
What can be concluded from the above activity?
A) Oxygen is 1/5th the volume of air.
B) The ratio of oxygen and nitrogen in air is 1 : 4.
C) Oxygen is required for burning the candle.
D) All of the above
Correct Answer: D
Solution :
From the given activity, we can conclude that (i) water level in the gas jar is 175th of the jar's volume which shows that the ratio of \[{{O}_{2}}\] is 175th the volume of air. (ii) this also proves that the ratio of \[{{O}_{2}}\] and \[{{N}_{2}}\] in the air is approximately 1:4. (iii) oxygen is required for burning because the flame of the candle goes off when \[{{O}_{2}}\]is used up.You need to login to perform this action.
You will be redirected in
3 sec
