A) \[{{T}_{1}}+{{T}_{2}}\]
B) \[\frac{({{T}_{1}}+{{T}_{2}})}{2}\]
C) \[\frac{{{T}_{1}}{{T}_{2}}({{p}_{1}}{{V}_{1}}+{{p}_{2}}{{V}_{2}})}{{{p}_{1}}{{V}_{1}}{{T}_{2}}+{{p}_{2}}{{V}_{2}}{{T}_{1}}}\]
D) \[\frac{{{T}_{1}}{{T}_{2}}({{p}_{1}}{{V}_{1}}+{{p}_{2}}{{V}_{2}})}{{{p}_{1}}{{V}_{1}}{{T}_{1}}+{{p}_{2}}{{V}_{2}}{{T}_{2}}}\]
Correct Answer: C
Solution :
| [c] |
| |
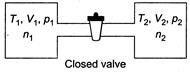 |
| From ideal gas equation, |
| (as P and T will become same) |
| |
| |
 |
| |
| |
| |
| Internal energy of the system (air in two vessels) remains same, before and after opening of valve, so |
| |
| where, f is degree of freedom of gas molecules |
| |
| |
| |
| |
| From Eq. (i) we can find the equilibrium pressure also. |
You need to login to perform this action.
You will be redirected in
3 sec
