Answer:
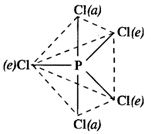
In \[PC{{l}_{5}}\]molecule, there are two types of bonds. These are \[P-Cl(e)\]bonds called equatorial (e) bonds and \[P-Cl(a)\]bonds called axial bonds. Now, the electron pair of axial bond is experiencing repulsion from the electron pairs of three equatorial bonds \[[P-F(e)]\] bonds at right angles to it in space. At the same time, the electron pair of equatorial bond is under repulsion from the electron pairs of two axial bonds \[[P-F(a)]\] at right angle to it. Thus, because of greater repulsion, the bond length of \[P-F(a)\]bond is slightly more than that of \[P-F(e)\]bond.
You need to login to perform this action.
You will be redirected in
3 sec
