Answer:
These amines can distinguished by Liebermann nitrosoamine reaction and Hinsberg's reagent test.
(i) Liebermann nitrosoamine reaction. N-methylpropan-2-amine being a \[2{}^\circ \] amine reacts with \[HN{{O}_{2}}(NaN{{O}_{2}}+HCl)\]at 273-278 K to give yellow coloured oily N-methyl-N-nitrosopropan-2 -amine. This, on warming with a crystal of phenol and conc. \[{{H}_{2}}S{{O}_{4}}\]gives a green solution which when made alkaline with aqueous \[NaOH\] turns deep blue and then red on dilution. This is called Liebermann nitrosoamine reaction.
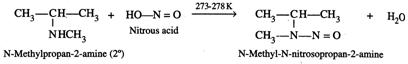 On the other hand, N-ethyl-N-methylethanamine being a
On the other hand, N-ethyl-N-methylethanamine being a ![]() amine does not give this test.
amine does not give this test.
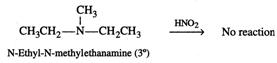 (ii) Hinsberg's reagent test. N-Methylpropan-2-amine being a \[2{}^\circ \] amine, on treatment with Hinsberg's reagent, i.e., benzenesulphonyl chloride \[({{C}_{6}}{{H}_{5}}S{{O}_{2}}Cl)\] forms N-isopropyl-N-methylbenzenesulphonainide which does not dissolve in aqueous NaOH due to absence of a H atom on the N-atom,
(ii) Hinsberg's reagent test. N-Methylpropan-2-amine being a \[2{}^\circ \] amine, on treatment with Hinsberg's reagent, i.e., benzenesulphonyl chloride \[({{C}_{6}}{{H}_{5}}S{{O}_{2}}Cl)\] forms N-isopropyl-N-methylbenzenesulphonainide which does not dissolve in aqueous NaOH due to absence of a H atom on the N-atom,
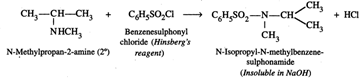 On the other hand, N-ethyl-N-methylethanamine being a \[3{}^\circ \] amine does not react with Hinseberg's reagent and hence can be easily distilled off from the reaction mixture.
On the other hand, N-ethyl-N-methylethanamine being a \[3{}^\circ \] amine does not react with Hinseberg's reagent and hence can be easily distilled off from the reaction mixture.
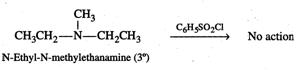
You need to login to perform this action.
You will be redirected in
3 sec
