Answer:
Since halogens are more
electronegative than carbon and also possess lone pairs of electrons,
therefore, they exert both -\[I\]- and +R- effects. Now in F, the lone pairs of
electrons are present in 2p-orbitals but in \[Cl,\] they are present in
3p-orbitals. Since 2p-orbitals of F and C are of almost equal size, therefore,
the +R-effect is more pronounced in p-fluorobenzoic acid than in
p-chlorobenzoic acid
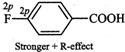
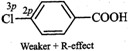 Thus, in p-fluorobenzoic acid,
+R-effect outweighs the -\[I\]-effect but is p-chlorobenzoic acid, it is the -\[I\]-effect
which outweighs the +R-effect. Consequently, p-fluorobenzoic acid is a weaker
acid than p-chlorobenzoic acid.
Thus, in p-fluorobenzoic acid,
+R-effect outweighs the -\[I\]-effect but is p-chlorobenzoic acid, it is the -\[I\]-effect
which outweighs the +R-effect. Consequently, p-fluorobenzoic acid is a weaker
acid than p-chlorobenzoic acid.
You need to login to perform this action.
You will be redirected in
3 sec
