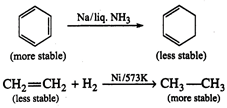
Answer:
The direction of the dipole moments
as well as the hybridisation states of the carbon atoms are shown as follows:

 The order of the electro negativity
of the carbon atoms based on hybridisation is\[sp>s{{p}^{2}}>s{{p}^{3}}\]
This means that \[C{{H}_{3}}C{{H}_{2}}-C\equiv
C\,-(s{{p}^{3}}-sp)\]bond is more polar than that of\[C{{H}_{3}}C{{H}_{2}}-CH=CH-(s{{p}^{3}}-s{{p}^{2}})\]
bond. Therefore, the dipole moment of but-1-yne is expected to be more than
that of but-1-ene.
The order of the electro negativity
of the carbon atoms based on hybridisation is\[sp>s{{p}^{2}}>s{{p}^{3}}\]
This means that \[C{{H}_{3}}C{{H}_{2}}-C\equiv
C\,-(s{{p}^{3}}-sp)\]bond is more polar than that of\[C{{H}_{3}}C{{H}_{2}}-CH=CH-(s{{p}^{3}}-s{{p}^{2}})\]
bond. Therefore, the dipole moment of but-1-yne is expected to be more than
that of but-1-ene.
You need to login to perform this action.
You will be redirected in
3 sec
