Answer:
We know that hydrochloric acid (HCl) is a
strong acid and acetic acid (CH3COOH) is a weak acid. Being a strong
acid, HC1 solution contains a much greater amount of hydrogen ions in it. Due
to this the fizzing will occur more vigorously in test tube A. The fizzing is
due to the evolution of H2 gas which is formed by the action of acid
on the magnesium ribbon.
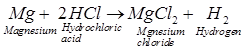
![]()
You need to login to perform this action.
You will be redirected in
3 sec
