Molecular Masses of Polymers
Category : JEE Main & Advanced
A polymer sample contains chain of varying lengths and therefore its molecular mass is always expressed as an average on the other hand natural polymer such as proteins contain chain of identical length and therefore they have definite molecular mass.
The molecular mass of a polymer can be expressed in two ways.
(1) Number average molecular mass \[({{\bar{M}}_{N}})\]\[\]
(2) Weight average molecular mass \[({{\bar{M}}_{W}})\].
(1) Number average molecular mass \[({{\bar{M}}_{N}})\] : If \[{{N}_{1}},\,{{N}_{2}},\,{{N}_{3}}\]….. are the number of molecules with molecular masses \[{{M}_{1}},{{M}_{2}},\,{{M}_{3}}\]…… respectively, then the number average molecular mass is
\[{{\bar{M}}_{N}}=\frac{{{N}_{1}}{{M}_{1}}+{{N}_{2}}{{M}_{2}}+{{N}_{3}}{{M}_{3}}+...}{{{N}_{1}}+{{N}_{2}}+{{N}_{3}}...}\]
This may be expressed as : \[{{\bar{M}}_{N}}=\frac{\sum {{N}_{i}}{{M}_{i}}}{\sum {{N}_{i}}}\]
Where \[{{N}_{i}}\] is the number of molecules of the ith type with molecular mass \[{{M}_{i}}\].
(2) Weight average molecular mass \[({{\bar{M}}_{W}})\] : If \[{{m}_{1}},\,{{m}_{2}},\,{{m}_{3}}\]…. are the masses of species with molecular masses \[{{M}_{1}},\,{{M}_{2}},\,{{M}_{3}}\]….. respectively, then the weight average molecular mass is
\[{{\bar{M}}_{W}}=\frac{{{m}_{1}}{{M}_{1}}+{{m}_{2}}{{M}_{2}}+{{m}_{3}}{{M}_{3}}....}{{{m}_{1}}+{{m}_{2}}+{{m}_{3}}+...}\] or \[=\frac{\sum {{m}_{i}}{{M}_{i}}}{\sum {{m}_{i}}}\]
But \[{{m}_{i}}={{N}_{i}}{{M}_{i}}\], so that \[{{\bar{M}}_{W}}=\frac{\sum {{N}_{i}}M_{i}^{2}}{\sum {{N}_{i}}{{M}_{i}}}\]
where \[{{N}_{i}}\] is the number of molecules of mass \[{{M}_{i}}\].
\[PDI=\frac{{{{\bar{M}}}_{W}}}{{{{\bar{M}}}_{n}}}\]
This gives an idea about the homogeneity of a polymer.
(i) The polymers whose molecules have nearly same molecular masses are called monodisperse polymers. For these molecules, \[{{\bar{M}}_{W}}={{\bar{M}}_{N}}\] and therefore, PDI is one.
(ii) The polymers whose molecules have wide range of molecular masses are called polydisperse polymers. For these polymers, \[{{\bar{M}}_{W}}>{{\bar{M}}_{N}}\] and therefore, their PDI is greater than one.
Thus, it may be concluded that in general, natural polymers are more homogeneous than synthetic polymers.
For natural polymers, PDI is usually unity and therefore, natural polymers are monodisperse.
For synthetic polymers, the PDI is greater than one and therefore \[{{\bar{M}}_{W}}\] is always greater than \[{{\bar{M}}_{N}}\]. \[{{\bar{M}}_{N}}\] is always determined by employing methods which depend upon the number of molecules present in the polymer sample. For example, colligative property such as osmotic pressure is used. On the other hand, weight average molecular mass is measured by using the methods such as light scattering and ultracentrifugation, sedimentation, etc. which depend upon the mass of individual molecules.
(3) Polymer in increasing order of their intermolecular forces are polythene < Buna S < Nylon-66.
(4) We always use purest monomer in free radical polymerisation reaction because the impurities can act as chain transfer agent and may combine with the free radical to slow down the reaction or even stop the reaction.
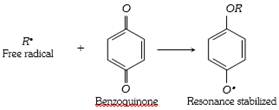
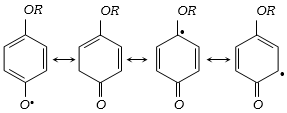
(5) Benzoquinone inhibit the free radical polymerisation of vinyl derivative because it combine with free radical intermediate to form a non reactive radical which is highly stabilized by resonance because of the lack of reactivity of the new radical formed, it inhibit the further progress of the chain reaction. Therefore the reaction stops.
(6) A thin film of polyester is known as Mylar film.
(7) PET plastic commonly used for soft drink bottles, transparent jars and bottles for use in kitchen are made up of polyethylene terephthalate.
(8) Glyptal resins or Alkyd resins obtained from ethylene glycol and phthalic acid are thermoplastic. However, resins obtained from glycerol and phthalic acid are thermosetting polymers, due to the formation of cross-links by the third –OH group present in glycerol.
(9) Thermosetting plastics are also called heat setting plastics whereas thermoplastics are called cold setting plastics.
(10) Latex is a colloidal dispersion of rubber in water. It is not a colloidal solution of isoprene in water or any other solvent.
(11) Polymerisation of isoprene by free radical mechanism (in the presence of Na and heat) gives a product which is different from natural rubber (Natural rubber is a polymer of isoprene). The synthetic product so obtained is a mixture of cis and trans configurations and resembled Gutta percha. Gutta percha is a naturally occurring polymer in plants. It is all trans-stereoisomer and is non-elastic.
(12) Terylene is a British name of Dacron.
(13) Co-polymer of vinyl chloride 90% and vinyl acetate 10% is called VINYON.
(14) Co-polymer of acrylonitrile 40% and vinyl chloride 60% is called DYNEL.
(15) Co-polymer of vinyl chloride and vinyledene chloride is called SARAN.
(16) Plasticizers cannot convert a thermosetting polymer into thermoplastic one. It converts a hard and brittle plastic into soft and easily pliable one at room temperature.
(17) Free radical polymerisation of isoprene do not give Gutta percha (Gutta percha is a natural polymer). The synthetic product so obtained resembles Gutta percha.
(18) Co-ordination polymerisation of isoprene gives a product similar to natural rubber.
(19) Latex is not a colloidal dispersion of isoprene in water.
You need to login to perform this action.
You will be redirected in
3 sec
