Answer:
The postulates of Bohr's model of atom are as follows
(i) In an atom, the electrons
revole around the nucleus in certain definite circular paths called orbits or
shells. These are represented by the letters K, L, M, N....... or the numbers n
= 1, 2, 3, 4 ...........
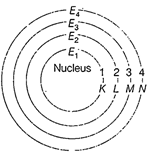 Circular orbits or energy levels or shells around the nucleus
(ii) The maximum number of electrons present in a shell is
given by the formula
Circular orbits or energy levels or shells around the nucleus
(ii) The maximum number of electrons present in a shell is
given by the formula ![]() .
Where 'n' is the orbit number or energy level index, 1, 2, 3 ...... Hence, the
maximum number of electrons in different shells are as follows.
First orbit (K shell) will be = 2 x 12 =2,
second orbit (or L shell) will be = 2 x22 = 8, third orbit (M shell)
will be = 2 x 32 = 18 and so on.
(iii) The maximum number of electrons that can be accommodated
in the outermost orbit is 8.
(iv) Electrons are not accommodated in a given shell,
unless the inner shells are filled. That is the shells are filled in a
step-wise manner,
(v) While revolving in discrete orbits the electrons do
not radiate energy.
.
Where 'n' is the orbit number or energy level index, 1, 2, 3 ...... Hence, the
maximum number of electrons in different shells are as follows.
First orbit (K shell) will be = 2 x 12 =2,
second orbit (or L shell) will be = 2 x22 = 8, third orbit (M shell)
will be = 2 x 32 = 18 and so on.
(iii) The maximum number of electrons that can be accommodated
in the outermost orbit is 8.
(iv) Electrons are not accommodated in a given shell,
unless the inner shells are filled. That is the shells are filled in a
step-wise manner,
(v) While revolving in discrete orbits the electrons do
not radiate energy.
You need to login to perform this action.
You will be redirected in
3 sec
