Answer:
If we take into account
the nuclear motion, the stationary state energies will be given by
![]() Let
Let ![]() be reduced mass of hydrogen
be reduced mass of hydrogen![]() , and
, and ![]() be
reduced mass of deuterium (H2). The frequency of 1st line
of Lyman series in hydrogen is given by
be
reduced mass of deuterium (H2). The frequency of 1st line
of Lyman series in hydrogen is given by
![]()
![]()
![]()
![]()
![]() The percentage difference
in wave number is
The percentage difference
in wave number is ![]()
![]()
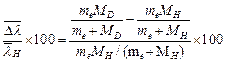 AS me
<<MH<MD
AS me
<<MH<MD
![]()
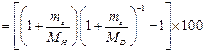 =
=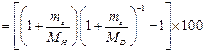
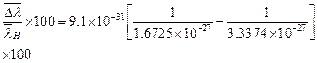
You need to login to perform this action.
You will be redirected in
3 sec
