Answer:
Packing efficiency is the percentage
of the total space which is occupied by the particles in a certain packing. The
fraction of the total space filled by spheres is called packing fraction.
Packing fraction.
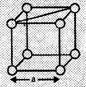
![]() (a) In a simple cubic
unit cell,
Suppose the edge
length of the unit cell = a
and radius of the
sphere = r
As spheres are
touching each other so, a = 2r
(a) In a simple cubic
unit cell,
Suppose the edge
length of the unit cell = a
and radius of the
sphere = r
As spheres are
touching each other so, a = 2r
 No. of spheres per
unit cell
No. of spheres per
unit cell ![]() Volume of 1 sphere
Volume of 1 sphere ![]() Volume of the cube
= a3 = (2r)3 = 8r3
Volume of the cube
= a3 = (2r)3 = 8r3
![]() Fraction
occupied i.e. packing fraction
Fraction
occupied i.e. packing fraction
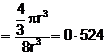 or % of space
occupied = 52
or % of space
occupied = 52![]() 4%
Percentage of
unoccupied space
= 100 ? 52
4%
Percentage of
unoccupied space
= 100 ? 52 ![]() 4 = 47
4 = 47 ![]() 6%
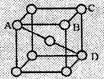
(b) In body
centered cubic structure,
As the sphere at
the centre touches the spheres at the corner, body diagonal AD = 4r
Further face
diagonal,
6%
(b) In body
centered cubic structure,
As the sphere at
the centre touches the spheres at the corner, body diagonal AD = 4r
Further face
diagonal,
![]() and body diagonal,
and body diagonal,
![]()
![]()
![]() Volume of the unit
cell
Volume of the unit
cell ![]() No. of spheres per
unit cell
No. of spheres per
unit cell ![]()
 Volume of 2 spheres
Volume of 2 spheres
![]()
![]() Fraction
occupied i.e. packing fraction
Fraction
occupied i.e. packing fraction
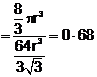 or % of space
occupied = 68%
Percentage of
unoccupied space
=100 ? 68 = 32%
(c) In face-centred
cubic structure
or % of space
occupied = 68%
Percentage of
unoccupied space
=100 ? 68 = 32%
(c) In face-centred
cubic structure
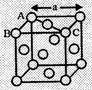 As spheres on the
face are touching, so AC = 4r
But from the right
angled triangle ABC,
As spheres on the
face are touching, so AC = 4r
But from the right
angled triangle ABC,
![]()
![]() or
or ![]()
![]() Volume of the unit
cell
Volume of the unit
cell
![]() No. of spheres in
the unit cell
No. of spheres in
the unit cell
![]() Volume of 4 spheres
Volume of 4 spheres![]() Fraction occupied
i.e. packing fraction or efficiency of packing
Fraction occupied
i.e. packing fraction or efficiency of packing
![]() or % of space
occupied = 74%
Percentage of
unoccupied space
= 100 ? 74 = 26%
or % of space
occupied = 74%
Percentage of
unoccupied space
= 100 ? 74 = 26%
You need to login to perform this action.
You will be redirected in
3 sec
