| Column 1 | Column 11 | |
| A. B. C. D. | Mg in solid state MgCI2 in molten state Silicon with phosphorus Germanium with boron | 1. p - type semiconductor 2. n -type semiconductor 3. Electrolytic conductors 4. Electronic conductors |
Answer:
A. ® (4) B.®
(3) C.® (2) D. ® (1)
A. Mg in solid state show electronic
conductivity due to presence of free electrons hence, they are known as
electronic conductors.
B. MgCl2 in molten
state show electrolytic conductivity due to presence of electrolytes in
molten state.
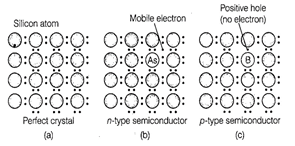
C. Silicon doped with phosphorus
contain one extra electron due to which it shows conductivity under the
influence of electric field and known as p-type semiconductor.
D. Germanium doped with boron
contain one hole due to which it 'shows conductivity under the influence of
electric field and known as n-type semiconductor.
You need to login to perform this action.
You will be redirected in
3 sec
