Answer:
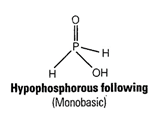
(c) Strong reducing behaviour of ![]() is
due to presence of two P ? H bonds and one P ? OH bond
is
due to presence of two P ? H bonds and one P ? OH bond
You need to login to perform this action.
You will be redirected in
3 sec
