Answer:
Due to greater
electronegativity and small size of O than S, H2O undergoes
extensive intermolecular H-bonding. As a result, H2O exists as an
associated molecule. Therefore, H2O is a liquid at room temperature.
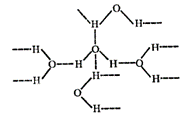 On the other hand,
H2S does not undergo H-bonding and exists as discrete molecules held
together by weak van der Waal's forces of attraction. Therefore, H2S
is a gas at room temperature.
On the other hand,
H2S does not undergo H-bonding and exists as discrete molecules held
together by weak van der Waal's forces of attraction. Therefore, H2S
is a gas at room temperature.
You need to login to perform this action.
You will be redirected in
3 sec
