Answer:
Magnetic moment ![]() When n =1,
When n =1,![]() =
= ![]() When n = 2,
When n = 2, ![]() When n = 3,
When n = 3, ![]() When n = 4,p =
When n = 4,p = ![]() When n = 5,
When n = 5, ![]() (i)
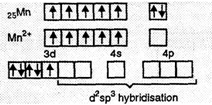
(i) ![]() . In this complex Mn
is in +2 oxidation state, (Mn2+ )
. In this complex Mn
is in +2 oxidation state, (Mn2+ )
![]() = 2.2 BM suggests
that it has only one unpaired electron. Thus, when CN? ligands
approach Mn2+ ion, the electrons in 3d subshell pair up as shown in
Fig. (a)
Thus, CN?
is a strong ligand.
d2 sp3hybridisation
is involved and the complex is inner orbital octahedral complex.
= 2.2 BM suggests
that it has only one unpaired electron. Thus, when CN? ligands
approach Mn2+ ion, the electrons in 3d subshell pair up as shown in
Fig. (a)
Thus, CN?
is a strong ligand.
d2 sp3hybridisation
is involved and the complex is inner orbital octahedral complex.
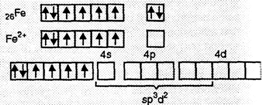
 (ii) [Fe(H2O)6]2+.
In this complex, Fe is in + 2 state (Fe2+).
(ii) [Fe(H2O)6]2+.
In this complex, Fe is in + 2 state (Fe2+).
![]() BM suggests that
there are four unpaired electrons. This means that the electrons in 3d do not
pair up when the ligands, H2O molecules, approach. Thus, H2O
is a weak ligand. To accommodate the electrons donated by the H2O
molecules, the hybridization involved will be sp3d2.
Hence, is will be outer orbital octahedral complex.
BM suggests that
there are four unpaired electrons. This means that the electrons in 3d do not
pair up when the ligands, H2O molecules, approach. Thus, H2O
is a weak ligand. To accommodate the electrons donated by the H2O
molecules, the hybridization involved will be sp3d2.
Hence, is will be outer orbital octahedral complex.
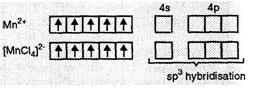
 (iii) K2[MnCl4].
In
this complex, Mn is in in + 2 state (Mn2+)
(iii) K2[MnCl4].
In
this complex, Mn is in in + 2 state (Mn2+) ![]() BM
suggests that there are five unpaired electrons. The hybridization is sp3
and the complex has tetrahedral geometry.
BM
suggests that there are five unpaired electrons. The hybridization is sp3
and the complex has tetrahedral geometry.
You need to login to perform this action.
You will be redirected in
3 sec
