Answer:
The
given haloarene is the resonance hybrid of the following structures :
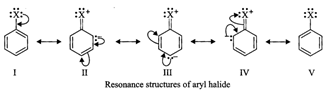 Since in the resonance structures (13-IV), the negative charge appears at
o- and ^-positions, therefore, the halogen atom (X) is o, p-directing and not
w-directing.
Since in the resonance structures (13-IV), the negative charge appears at
o- and ^-positions, therefore, the halogen atom (X) is o, p-directing and not
w-directing.
You need to login to perform this action.
You will be redirected in
3 sec
