Answer:
(i) (ii)
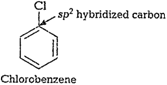
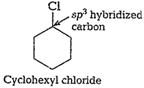 Due to
Due to ![]() hybridization
of C-atom in chlorobenzene, C-atom ismore electronegative (greater s-character)
whereas in cyclohexylchloride, C-atom is
hybridization
of C-atom in chlorobenzene, C-atom ismore electronegative (greater s-character)
whereas in cyclohexylchloride, C-atom is ![]() hybridized,
i.e., less electronegative (lessers-character). So polarity of
hybridized,
i.e., less electronegative (lessers-character). So polarity of ![]() bond
in chlorobenzene is lessthan the
bond
in chlorobenzene is lessthan the ![]() bond
in cyclohexyl chloride. Therefore, the dipolemoment of chlorobenzene is lower
than that of cyclohexylchloride.
(ii) Water molecules have enough
strong H-bonding (intermolecular)which is difficult to be broken by alkyl
halides, though they arealso polar in nature. Therefore, alkyl halides do not
dissolve inwater and form separate layers.
(iii) Grignard reagents (R?Mg?X)
are readily decomposed by waterto produce alkanes. That is why they should be
prepared underanhydrous conditions. Instead, ether is used as a solvent
duringthe preparation of Grignard reagent.
bond
in cyclohexyl chloride. Therefore, the dipolemoment of chlorobenzene is lower
than that of cyclohexylchloride.
(ii) Water molecules have enough
strong H-bonding (intermolecular)which is difficult to be broken by alkyl
halides, though they arealso polar in nature. Therefore, alkyl halides do not
dissolve inwater and form separate layers.
(iii) Grignard reagents (R?Mg?X)
are readily decomposed by waterto produce alkanes. That is why they should be
prepared underanhydrous conditions. Instead, ether is used as a solvent
duringthe preparation of Grignard reagent.
![]()
You need to login to perform this action.
You will be redirected in
3 sec
