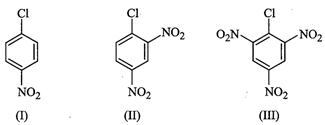
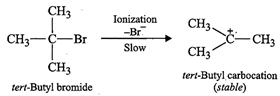
Answer:
tert-Butyl
bromide readily loses ![]() ion to form stable
fert-butyl carbocation. Therefore, it undergoes reaction by
ion to form stable
fert-butyl carbocation. Therefore, it undergoes reaction by ![]() mechanism which occurs in two
steps. In the first step, tert-butyl carbocation is formed. This
step is slow and hence is the rate determining step of the
reaction. In the second step, the tert-butyl carbocation is readily attacked
by
mechanism which occurs in two
steps. In the first step, tert-butyl carbocation is formed. This
step is slow and hence is the rate determining step of the
reaction. In the second step, the tert-butyl carbocation is readily attacked
by ![]() ion to form tert-butyl
alcohol.
ion to form tert-butyl
alcohol.
You need to login to perform this action.
You will be redirected in
3 sec
