Answer:
(b, c)
Left side of cell reaction represents
oxidation half cell i.e., oxidation of Mg and right side of cell represents
reduction half cell reactions i.e., reduction of copper.
(ii) Cu is reduced and reduction
occurs at cathode.
(iii) Mg is oxidised and oxidation
occurs at anode.
(iv) whole cell reaction can be
written as
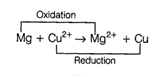 Hence, options (b) and (c) both
are correct choices.
Hence, options (b) and (c) both
are correct choices.
You need to login to perform this action.
You will be redirected in
3 sec
