Answer:
(a)
![]() is a weak field
ligand.
is a weak field
ligand.
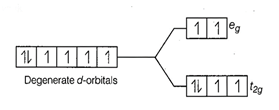 Configuration of
Configuration of ![]() Number of unpaired electrons (n) = 4
Magnetic moment
Number of unpaired electrons (n) = 4
Magnetic moment
![]()
![]()
![]() is a weak field
ligand.
is a weak field
ligand.
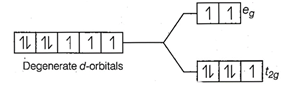 Configuration of
Configuration of ![]() Number of unpaired electrons (n) = 3
Number of unpaired electrons (n) = 3
![]()
![]()
![]() CN is strong field ligand.
CN is strong field ligand.
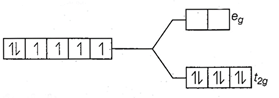
![]() There is no unpaired electron, so it is diamagnetic.
There is no unpaired electron, so it is diamagnetic.
![]() =
0
(b)
=
0
(b) ![]()
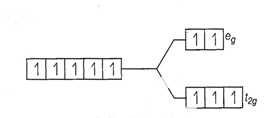
![]() Number of unpaired electrons, n = 5
Number of unpaired electrons, n = 5
![]()
![]()
![]()
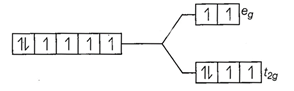
![]() Number of unpaired electrons, n = 4
Number of unpaired electrons, n = 4
![]()
![]() = 4.98 BM
= 4.98 BM
![]()
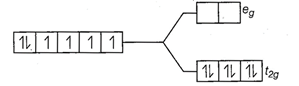 Since,
Since, ![]() is a
strong field ligand, all the electrons get paired.
is a
strong field ligand, all the electrons get paired.
![]() Because there is no unpaired electron, so it is diamagnetic
in nature.
Because there is no unpaired electron, so it is diamagnetic
in nature.
You need to login to perform this action.
You will be redirected in
3 sec
