| Column I (Complex ion) | Column II (Hybridisation, number of unpaired electrons) |
|
A. |
1. |
Answer:
A.®(3)
B.®(1) C.®(4) D.®(2)
Formation of inner orbital complex and outer orbital
complex determines hybridisation of molecule which in turn depends upon field
strength of ligand and number of vacant d orbitals.
(i) Strong field ligand forms inner orbital complex with
hybridisation ![]() .
Weak field ligand forms outer orbital complex with
hybridisation
.
Weak field ligand forms outer orbital complex with
hybridisation ![]() .
According to VBT, hybridisation and number of unpaired
electrons of coordination compounds can be calculated as
.
According to VBT, hybridisation and number of unpaired
electrons of coordination compounds can be calculated as
![]() MOEC (Molecular orbital electronic configuration) of
MOEC (Molecular orbital electronic configuration) of ![]() in
in ![]() is
is
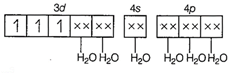 Hybridisation = d2sp3
n (number of unrpaired
electrons) = 3
B.
Hybridisation = d2sp3
n (number of unrpaired
electrons) = 3
B. ![]() MOEC of
MOEC of ![]() is
is
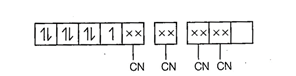 Hybridisation =
Hybridisation = ![]() n = 1.
c.
n = 1.
c. ![]() MOEC of
MOEC of ![]() in
in ![]() is.
is.
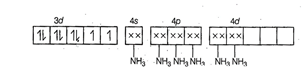 Hybridisation =
Hybridisation = ![]() n = 2
D.
n = 2
D. ![]() MOEC of
MOEC of ![]() in
in ![]() is
is
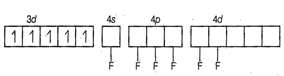 Hybridisation =
Hybridisation = ![]() n = 5
Hence, correct choice can be represented by (a).
n = 5
Hence, correct choice can be represented by (a).
You need to login to perform this action.
You will be redirected in
3 sec
