Answer:
(a) Three isomers.
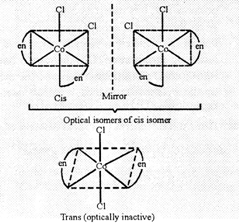 (b) Cis and trans
are geometrical isomers.
(b) Cis and trans
are geometrical isomers.
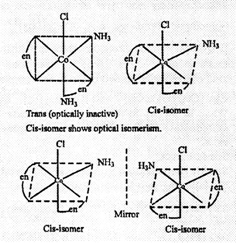 Thus, three isomers
are possible.
(c) Three isomers
Cis and trans are
geometrical isomers
Cis form is
optically active as it has no plane of symmetry.
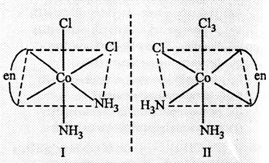
Thus, three isomers
are possible.
(c) Three isomers
Cis and trans are
geometrical isomers
Cis form is
optically active as it has no plane of symmetry.
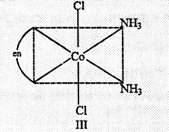 Trans isomer is
optically inactive because of the presence of a plane of symmetry.
Trans isomer is
optically inactive because of the presence of a plane of symmetry.
You need to login to perform this action.
You will be redirected in
3 sec
