Answer:
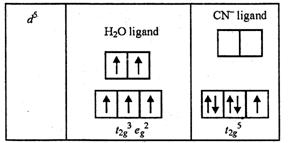
Ans. Mn in +2
state has the configuration 3d5. In present of H2O which
is weak ligand, the distribution of these five electrons is ![]() i.e.,
all the electrons remain unpaired. In presence of CN? which is a
strong ligand, the distribution is
i.e.,
all the electrons remain unpaired. In presence of CN? which is a
strong ligand, the distribution is ![]() i.e.,
two t2g orbitals contain paired electrons while the third
i.e.,
two t2g orbitals contain paired electrons while the third ![]() orbital contains
one unpaired electron.
orbital contains
one unpaired electron.
You need to login to perform this action.
You will be redirected in
3 sec
