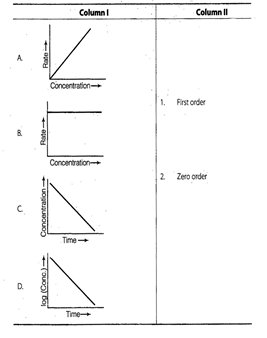
Answer:
A.®(1) B.® (2) C.® (2) D.®(1)
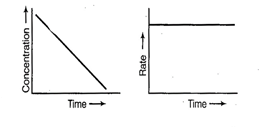
For zero order reaction rate equation
may be written as
![]() Which denotes a straight line
equation similar to y = m x + c
On transforming (i)
Which denotes a straight line
equation similar to y = m x + c
On transforming (i) ![]()
![]()
![]()
![]() For a first order reaction
For a first order reaction
![]() [Concentration]1
[Concentration]1
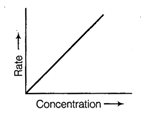
![]() Graph between rate and
concentration may be drawn as
Graph between rate and
concentration may be drawn as
![]()
![]()
![]()
![]()
You need to login to perform this action.
You will be redirected in
3 sec
