Answer:
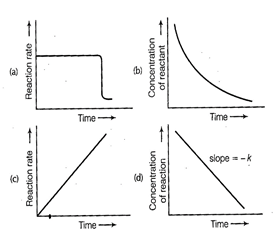
(a, d)
For a zero order reaction
![]() On comparing with Eq. of straight
line
On comparing with Eq. of straight
line
![]() x = t time
Slope (m) = - k rate constant
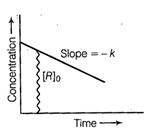
Intercept (c) = [R]0
initial concentration
x = t time
Slope (m) = - k rate constant
Intercept (c) = [R]0
initial concentration
 On rearranging Eq. (i)
On rearranging Eq. (i)
![]()
![]()
![]()
You need to login to perform this action.
You will be redirected in
3 sec
