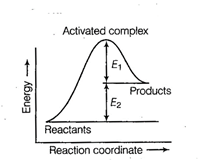
 (a) Activation energy of forward
reaction is
(a) Activation energy of forward
reaction is Answer:
(a) Activation energy is the minimum
energy required to convert reactant molecules to product molecules. Here, the
energy gap between reactants and activated complex is
sum of ![]() and
and ![]() .
-
.
-
![]() Activation energy =
Activation energy = ![]() Product is less stable than
reactant as energy of product is greater than the reactant.
Product is less stable than
reactant as energy of product is greater than the reactant.
You need to login to perform this action.
You will be redirected in
3 sec
