Answer:
A catalyst is a substance which increases
the speed of a reaction without itself undergoing any chemical change.
According to "intermediate complex
formation theory" reactants first combine with the catalyst to form an
intermediate complex which is short-lived and decomposes to form the products
and regenerating the catalyst.
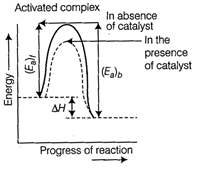
The intermediate formed has much
lower potential energy than the intermediate complex formed between the
reactants in the absence of the catalyst.
Thus, the presence of catalyst
lowers the potential energy barrier and the reaction follows a new alternate
pathway which require less activation energy.
We know that, lower the
activation energy, faster is the reaction because more reactant molecules can
cross the energy barrier and change into products.
Enthalpy, ![]() is a state function.
Enthalpy of reaction, i.e., difference in energy between reactants and product
is constant, which is clear from potential energy diagram.
Potential energy diagram of
catalysed reaction is given as
is a state function.
Enthalpy of reaction, i.e., difference in energy between reactants and product
is constant, which is clear from potential energy diagram.
Potential energy diagram of
catalysed reaction is given as
You need to login to perform this action.
You will be redirected in
3 sec
