Answer:
(i)
M.F. of compound A ![]() corresponds to the
general formula
corresponds to the
general formula ![]() where n = 4,
therefore, A is an alkene.
Since ozonolysis of compound A
where n = 4,
therefore, A is an alkene.
Since ozonolysis of compound A ![]() gives two
moles of acetaldehyde, therefore, alkene (A) must be symmetrical, i.e.,
but-2-ene
gives two
moles of acetaldehyde, therefore, alkene (A) must be symmetrical, i.e.,
but-2-ene
![]() (ii) Since compound (A), i.e., but-2-ene reacts with HCl
to form compound (B), therefore, (B) must be 2-chlorobutane.
(ii) Since compound (A), i.e., but-2-ene reacts with HCl
to form compound (B), therefore, (B) must be 2-chlorobutane.
![]()
![]() (iii) Since compound (B), i.e., 2-chlorobutane reacts with
one mole of
(iii) Since compound (B), i.e., 2-chlorobutane reacts with
one mole of ![]() to give compound (C),
therefore, compound (C) must be a 1° amine, i.e., butan-2-amine
to give compound (C),
therefore, compound (C) must be a 1° amine, i.e., butan-2-amine
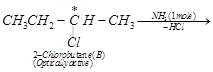
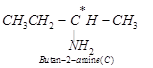 (iv) Since compound (C) reacts with
(iv) Since compound (C) reacts with ![]() to give an alcohol (D), therefore,
compound (D) must be butan-2-ol. Please note that 2-chlorobutane (B),
butan-2-amine (C) and butan-2-ol (D) contain a chiral carbon, therefore, all
these compounds are optically active.
to give an alcohol (D), therefore,
compound (D) must be butan-2-ol. Please note that 2-chlorobutane (B),
butan-2-amine (C) and butan-2-ol (D) contain a chiral carbon, therefore, all
these compounds are optically active.
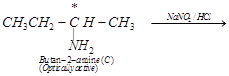

You need to login to perform this action.
You will be redirected in
3 sec
