Answer:
In
alkaline medium, phenol forms phenoxide ion which is more electron rich and hence
more reactive than phenol. Coupling reactions between diazonium salts is an
example of electrophilic aromatic substitution.
In this reaction, diazonium salt acts as the electrophile and phenoxide
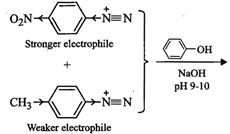
ion as the nucleophile. Evidently stronger the electrophile faster is the
reaction. Now due to electro-withdrawing effect of the ![]() group, p-nitrophenyldiazonium
cation is a stronger electrophile than p-toluenediazonium cation (+I-effect of
group, p-nitrophenyldiazonium
cation is a stronger electrophile than p-toluenediazonium cation (+I-effect of ![]() . group reduces its
electrophilicity) and hence couples preferentially with phenol to form
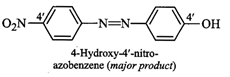
4-hydroxy-4'-nitroazobenzene as the major product.
. group reduces its
electrophilicity) and hence couples preferentially with phenol to form
4-hydroxy-4'-nitroazobenzene as the major product.
You need to login to perform this action.
You will be redirected in
3 sec
