Answer:
(i) Methylamine and dimethyl amine or isocyanide testMethyl
amine (primary) responds to carbyl amine reaction. Whenheated with chloroform
and KOH, it produces foul smell. Dimethylamine (secondary) does not respond to
this test.
![]() (ii) Secondary and tertiary amines
Libermann?s nitroso reactionSecondary amines (both aliphatic and aromatic)
react with nitrousacid to form nitrosoamines (yellow oily liquid)
(ii) Secondary and tertiary amines
Libermann?s nitroso reactionSecondary amines (both aliphatic and aromatic)
react with nitrousacid to form nitrosoamines (yellow oily liquid)
![]() When the yellow oily liquid is warmed
with a crystal of phenol and a few drops of cone.
When the yellow oily liquid is warmed
with a crystal of phenol and a few drops of cone. ![]() agreenish solution is
formed, It changes to red on dilution with water but changes to deep blue
onaddition of aqueous
agreenish solution is
formed, It changes to red on dilution with water but changes to deep blue
onaddition of aqueous ![]() solution.
Tertiary amines do not
give this test.
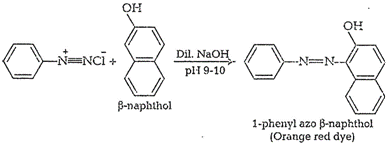
(iii) Ethyl amine and aniline
Ethyl amine (primary aliphatic amine)and aniline (primary aromatic amine) can
be distinguished by azodye test
Aniline responds to this test
whereas ethyl amine does not.
solution.
Tertiary amines do not
give this test.
(iii) Ethyl amine and aniline
Ethyl amine (primary aliphatic amine)and aniline (primary aromatic amine) can
be distinguished by azodye test
Aniline responds to this test
whereas ethyl amine does not.


 (iv) Aniline and benzyl amine
(nitrous acid test) Benzyl amine reactswith nitrous acid to form a
diazonium salt which is unstable. Itevolves
(iv) Aniline and benzyl amine
(nitrous acid test) Benzyl amine reactswith nitrous acid to form a
diazonium salt which is unstable. Itevolves ![]() gas by decomposing at
even low temperature.
gas by decomposing at
even low temperature.
![]()
![]() Aniline reacts with
nitrous acid to from benzene diazonium chloride which does not decompose to
give
Aniline reacts with
nitrous acid to from benzene diazonium chloride which does not decompose to
give![]() .
.
![]() (v) Aniline and N-methylaniline
(carbylamine test) Aniline givecarbylamine test because it is a primary
amine whereas N-methyl aniline (secondary amine) does not give this test.
(v) Aniline and N-methylaniline
(carbylamine test) Aniline givecarbylamine test because it is a primary
amine whereas N-methyl aniline (secondary amine) does not give this test.
![]()
You need to login to perform this action.
You will be redirected in
3 sec
