Answer:
The
functional isomers of the carbonyl compound with ![]() are :
are :
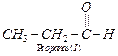
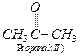 Due to less +I-effect of one
Due to less +I-effect of one ![]() group as compared to two
group as compared to two
![]() groups in
groups in ![]() and less steric hindrance,
compound I,
and less steric hindrance,
compound I,![]() will react faster with
HCN than compound n
will react faster with
HCN than compound n ![]() . For
mechanism, refer to sec. 12.6.1.3 pages 12/24-12/25.
The reaction will not proceed to completion since it is a
reversible reaction. Addition of strong acid inhibits the reaction because the
formation of
. For
mechanism, refer to sec. 12.6.1.3 pages 12/24-12/25.
The reaction will not proceed to completion since it is a
reversible reaction. Addition of strong acid inhibits the reaction because the
formation of ![]() from HCN is prevented.
from HCN is prevented.
You need to login to perform this action.
You will be redirected in
3 sec
