Answer:
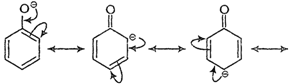
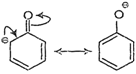
(i) Phenoxide ion
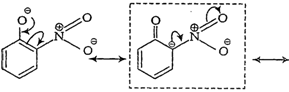
 (ii) o-nitrophenoxide ion
(ii) o-nitrophenoxide ion
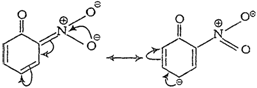
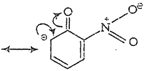 (iii) p-nitrophenoxide ion
(iii) p-nitrophenoxide ion
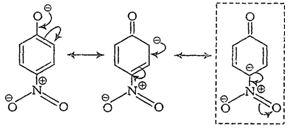
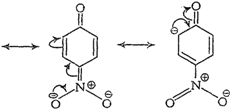 Due to ?R effect of ?
Due to ?R effect of ?![]() group, o- and p-nitrophenoxide ions are more stable than phenoxide ion.
Consequently, both o- and p-nitrophenols are more acidic than phenol.
Note Structures in boxes
have negative charge on that C-atom to which electron withdrawing ?
group, o- and p-nitrophenoxide ions are more stable than phenoxide ion.
Consequently, both o- and p-nitrophenols are more acidic than phenol.
Note Structures in boxes
have negative charge on that C-atom to which electron withdrawing ?![]() group is attached. So, these structures contribute more towards the acidic
character than the others.
group is attached. So, these structures contribute more towards the acidic
character than the others.
You need to login to perform this action.
You will be redirected in
3 sec
