Answer:
Due
to -1 and- R-effect of the ![]() group,
electron density in the O?H bond decreases while due to +1 or hyper conjugation
effect, of the
group,
electron density in the O?H bond decreases while due to +1 or hyper conjugation
effect, of the ![]() group, the
electron density in the O?H bond increases.
group, the
electron density in the O?H bond increases.
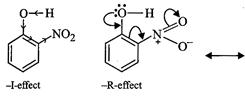
 As a result, O?H bond is o-nitrophenol is much weaker than the O?H bond
is o-cresol and hence o-nitrophenol is much more acidic than o-cresol.
As a result, O?H bond is o-nitrophenol is much weaker than the O?H bond
is o-cresol and hence o-nitrophenol is much more acidic than o-cresol.
You need to login to perform this action.
You will be redirected in
3 sec
