 (a)
(a)Answer:
(b)
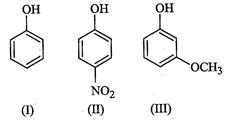
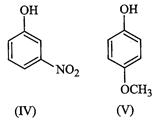
Since electron-withdrawing groups increase the acidity of phenols, therefore,
both p-nitrohenol (II) and m-nitrophenol (IV) are stronger acids than phenol
(I). However, due to the combined -I and -R effect of the ![]() group at p-position,
p-nitrophenol (II) is a much stronger acid than m-nitrophenol (IV), which
exerts only -I-effect at w-position.
Usually electron-donating groups decrease the acidity of phenols. But
group at p-position,
p-nitrophenol (II) is a much stronger acid than m-nitrophenol (IV), which
exerts only -I-effect at w-position.
Usually electron-donating groups decrease the acidity of phenols. But ![]() group at m-position cannot
exert its +R-effect but can exert only its -I-effect, therefore,
m-methoxyphenol (in) is a stronger acid than phenol (I). In contrast, at p-posidon,
group at m-position cannot
exert its +R-effect but can exert only its -I-effect, therefore,
m-methoxyphenol (in) is a stronger acid than phenol (I). In contrast, at p-posidon,
![]() group exerts its
+R-effect which makes p-methoxyphenol (V) a weaker acid than phenol (I). Thus,
overall acid strength of these five phenols decreases in the order: II > IV
> HI > I > V, i.e., option (b) is correct.
group exerts its
+R-effect which makes p-methoxyphenol (V) a weaker acid than phenol (I). Thus,
overall acid strength of these five phenols decreases in the order: II > IV
> HI > I > V, i.e., option (b) is correct.
You need to login to perform this action.
You will be redirected in
3 sec
