Answer:
Hydrogen bonding is present between the ethanol molecules
which is absent in the molecules of methoxymethane. So, a large amount of
energy is required to break these strong" bonds in ethanol.
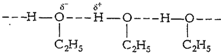
You need to login to perform this action.
You will be redirected in
3 sec
