Answer:
Nitro ![]() group is
electron withdrawing whereas methoxy
group is
electron withdrawing whereas methoxy![]() group is
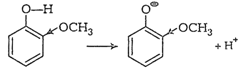
electron releasing in nature, o-nitrophenol produces
group is
electron releasing in nature, o-nitrophenol produces![]() ions
easily but methoxyphenol does not. This is becauseo-nitrophenoxide ion is
stabilised due to resonance. This is not true witho-methoxyphenoxide ion. The
two negative charges repel each otherthereby destabilising it.
ions
easily but methoxyphenol does not. This is becauseo-nitrophenoxide ion is
stabilised due to resonance. This is not true witho-methoxyphenoxide ion. The
two negative charges repel each otherthereby destabilising it.

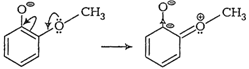 Note Also give resonating
structures of o-nitrophenoxide ion for Q. 8intext questions.
Note Also give resonating
structures of o-nitrophenoxide ion for Q. 8intext questions.
You need to login to perform this action.
You will be redirected in
3 sec
