Answer:
The mechanism involves 3 steps:
Step I: Protonation of alkene to form
carbocation by electrophile attack of ![]() .
.
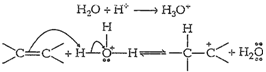 Step II: Nucleophilic
attack of water on carbocation formed.
Step II: Nucleophilic
attack of water on carbocation formed.
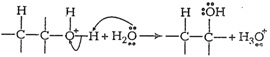
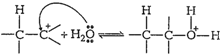 Step III: Deprotonation
to form corresponding alcohol.
Step III: Deprotonation
to form corresponding alcohol.
You need to login to perform this action.
You will be redirected in
3 sec
