Answer:
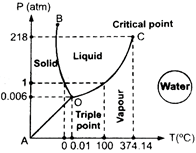 When pressure is
increased in solid state (at \[{{0}^{o}}C,\] 1 atm) ice change into liquid state
(water) while decreasing pressure in liquid state (at \[{{0}^{o}},\] 1 atm), water
changes to ice. When we squeeze the crushed ice, some of it melts into water
which fills the gaps between ice flakes. On the other hand, when pressure is
released, this water freezes into ice and binds together all the ice flakes to
make the ball more stable.
When pressure is
increased in solid state (at \[{{0}^{o}}C,\] 1 atm) ice change into liquid state
(water) while decreasing pressure in liquid state (at \[{{0}^{o}},\] 1 atm), water
changes to ice. When we squeeze the crushed ice, some of it melts into water
which fills the gaps between ice flakes. On the other hand, when pressure is
released, this water freezes into ice and binds together all the ice flakes to
make the ball more stable.
You need to login to perform this action.
You will be redirected in
3 sec
