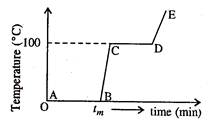
 (a) The region AB
represents ice and water in thermal equilibrium.
(b) At B water
starts boiling.
(c) At C all the
water gas converted into steam.
(d) C to D
represents water and steam in equilibrium at boiling point.
(a) The region AB
represents ice and water in thermal equilibrium.
(b) At B water
starts boiling.
(c) At C all the
water gas converted into steam.
(d) C to D
represents water and steam in equilibrium at boiling point.
Answer:
(a,
d)
(a)
In region AB, a phase change takes place from solid (ice) to liquid (water)
without a change of temperature. The heat supplied is used to increase the
energy of the molecules so that they can break loose from their bonds in the
solid structure and change into a liquid.
(d)
In the region CD, a phase change takes place a from liquid (water) to a vapour
state (steam) without a change in temperature. The heat supplied is used to
break loose the molecules of water free molecules in the steam phase.
You need to login to perform this action.
You will be redirected in
3 sec
