Answer:
(a) Blue colour of ![]() (aq)Solution
disappears
Reason Zinc reacts with a copper
sulphate to form colourless zinc sulphte solution and solid copper is deposited
fro, as Zn is more reactive than Cu, so, displaces Cu from
(aq)Solution
disappears
Reason Zinc reacts with a copper
sulphate to form colourless zinc sulphte solution and solid copper is deposited
fro, as Zn is more reactive than Cu, so, displaces Cu from ![]() solution
solution
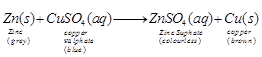 (b) Hydrogen gas is evolved Reason
Alumninum reacts with dilute hydrochloric acid to form aluminium chloride and
hydrogen gas.
(b) Hydrogen gas is evolved Reason
Alumninum reacts with dilute hydrochloric acid to form aluminium chloride and
hydrogen gas.
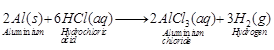 (c)No reaction occurs
Reason Silver metal is less reactive than
copper, therefore, it cannot displace copper from copper sulphate solution.
(c)No reaction occurs
Reason Silver metal is less reactive than
copper, therefore, it cannot displace copper from copper sulphate solution.
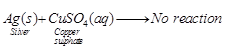
You need to login to perform this action.
You will be redirected in
3 sec
