Answer:
Activity:
To show that alcohols and glucose are not acids.
Materials
required: Dilute solution of ethanol and glucose solution Apparatus
required : Beaker (1), carbon electrodes (2), dry cells (2), bulb 1.5 V
(1),
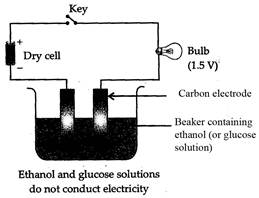 Procedure:
Take a beaker and place two carbon electrodes into it. Connect the electrodes
to a battery bulb through a key and a dry cell. Pour ethanol into the beaker
and press the key. See, if the bulb glows. Bulb does not glow. Repeat similar
experiment with glucose solution. Record your observations.
Observation
: It is observed that the bulb does not glow With both the solutions.
Conclusion
: The solutions of glucose and ethanol are non-conductors of electricity.
Explanation:
Ethanol and the solution of glucose containing hydrogen in their molecules do
not conduct electricity. Therefore/ these compounds do not produce
Procedure:
Take a beaker and place two carbon electrodes into it. Connect the electrodes
to a battery bulb through a key and a dry cell. Pour ethanol into the beaker
and press the key. See, if the bulb glows. Bulb does not glow. Repeat similar
experiment with glucose solution. Record your observations.
Observation
: It is observed that the bulb does not glow With both the solutions.
Conclusion
: The solutions of glucose and ethanol are non-conductors of electricity.
Explanation:
Ethanol and the solution of glucose containing hydrogen in their molecules do
not conduct electricity. Therefore/ these compounds do not produce ![]() ions in
solutions.
Hence these
are not categorised as acids.
ions in
solutions.
Hence these
are not categorised as acids.
You need to login to perform this action.
You will be redirected in
3 sec
